I have tried to cover the life story of Charles- Augstein de coulomb with the discovery of coulomb law.
The concept of coulomb's law was introduced by Charles-Augstein de coulomb in 1785. Did you know? Coulomb did not measure the attractive and repulsive force at the same time. He took one year to measure the attractive force after measuring of repulsive force.
The gravitational force equation discovered helped him to derive the coulombs equation
Curiosity to calculate electrostatic force
In 1777, While at the Paris Academy Charles-Augstein de coulomb took part in a competition to improve the sense of the compass by decreasing the friction, so he hung the magnetic needle on the silk string and rejected the friction altogether.
While working on that experiment, he noticed that the compass become more sensitive to attract in the direction of the electrostatic force.
From that moment, he decided to measure the force on the electrostatic charge by using the twisting force of the needle but the problem is that the electrostatic force is very small to measure. Most of the scales and balances cannot measure such minute forces. So he needs to invent one device.
So he decided to study the twisting force called torsional force because he already knows that the twisting force of the compass needle is dependent on the force of the electrostatic charge.
Background laws behind coulomb's law
While studying twisting force, he noticed that twisting items worked similarly to elastic properties such as springs and it is called hook's law.
Hooke's law is discovered by Robert Hooke in the year 1679. The law explained that stress is directly proportional to strain. He also proved it by mathematical equation by experimenting in spring.
Hooke's law experiment
The force required for stretching spring depends on the strength of the spring and is called the spring constant.
Hooke's law derivation,
Robert Hooke considered the mass as the force when he increased the stretching or length of the spring is also increased.
Fs= kx
Through Hooke's law experiment he invented the coulomb torsion balance device. This helped him to achieve what he needed.
Coulomb's Torsion balance or Torsion pendulum
The torsion pendulum device allowed him to measure the minute force of the charge. This is one of the world's precise measuring devices, where a degree on the scale was equivalent to 1/100,000th of the weight of a single grain of sand.
The torsion pendulum may look simple but the actual experiment was incredibly difficult to complete, and the machine was described by a contemporary as an "all too unsteady twisting machine". Still, the torsion machine was then used for all kinds of scientific experiments.
Coulomb's first paper
In 1785, Coulomb used his torsional balance on an electrically charged spherical ball and experimentally determined that the electrical repulsion force between the two same charged spherical balls is inversely proportional to the square of the distance between them.
He released his first paper on "Fundamental law of electricity" on that he explained about the repulsive force between two charged spherical ball.
He had little difficulty measuring the attractive force between two charged spherical balls. But the following year experimentally he proved that this attractive force follow the same rule and in 1786, he wrote
Coulomb's Law statement
The force, either repulsive or attractive, from two electrified objects...is due to the electricl fluid in the two electrical objects, and inverse of the square of the distance.
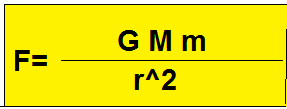
Electric force & Gravitational force
Coulomb was not the first person to predict that the electric forces look like gravitational force but he was the first to experimentally validate it and the law is justly named coulomb's law.
Electric force
Gravitational force
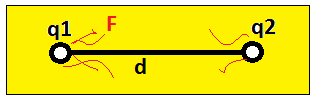
Statement of Coulomb's law
In figure q1 and q2 are two charges. F is the force exerted by two charges. Force exerts by charge is either attract or repel force.
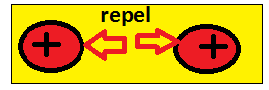
Coulomb's First law statement
- The same charges repel each other. (example:+ve and +ve or -ve and -ve charge)
- Opposite charges attract each other. (example:+ve and -ve charge)
Coulomb's Second law statement
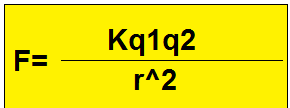
The force between two charges is directly proportional to the product of the two charges and inversely proportional to the square of the distance between them.
- F ∝ q1*q2
- F∝ 1/d^2
Coulomb's law derivation
F ∝ q1*q2
combining these two-equation, then the force will be,
While equating this K constant will come.
The value of K is
εo=Absolute Permittivity of air or vacuum.
εr=Relative permittivity of the medium.
(value of εr =1 for air or vacuum).
While simplifying this
F=(9*10^9*q1*q2)/(εr*d^2) for a medium
The absolute permittivity of air or vacuum
The absolute permittivity of air or vacuum is constant, not changeable anywhere in the earth and it is easy to memorize. It is denoted as epsilon Knot (εo) which is a Greek letter. The value of (εo) is equal to 8.854*10^-12. The Standard International unit for permittivity is expressed in farad/meter.
Limitation of coulomb's law
- We cannot apply Coulomb's law to moving charges because it is difficult to locate charge distances as it continues to move. The coulombs law is exactly consistent with a static charge.
- It is only applicable for charges in a straight line. we cannot apply coulombs law to the static charges with arbitrary form.
- From the coulombs law definition, we can clear that the coulombs law only obeys inverse square law.
- The distance between the two charges must be up to 10^-15 m because at this distance only force is strong.






Nice
ReplyDelete